Chapter 14 the behavior of gases answer key – Chapter 14: The Behavior of Gases Answer Key unveils the fundamental principles that govern the behavior of gases, providing a comprehensive understanding of their properties and interactions. This key offers invaluable insights into the kinetic molecular theory, gas laws, and the deviations of real gases from ideal behavior, empowering readers to predict and analyze gas behavior with precision.
Delving into the kinetic molecular theory, this key elucidates its postulates and demonstrates how it explains the behavior of gases. It explores Boyle’s law, Charles’s law, and the combined gas law, highlighting their significance in solving problems involving gases. Furthermore, it defines ideal gases, examines the assumptions of the ideal gas law, and derives it from the kinetic molecular theory.
1. Kinetic Molecular Theory: Chapter 14 The Behavior Of Gases Answer Key

Kinetic molecular theory (KMT) is a model of the behavior of gases that assumes that gas particles are in constant, random motion and that they collide with each other and the walls of their container.
The postulates of the KMT are as follows:
- Gas particles are in constant, random motion.
- Gas particles collide with each other and the walls of their container.
- The average kinetic energy of gas particles is proportional to the absolute temperature of the gas.
- The volume of a gas is proportional to the number of gas particles in the container.
- The pressure of a gas is proportional to the number of gas particles colliding with the walls of the container per unit time.
The KMT can be used to explain a wide variety of the behavior of gases, including:
- The pressure of a gas is proportional to the temperature of the gas.
- The volume of a gas is proportional to the temperature of the gas.
- The rate of effusion of a gas is inversely proportional to the square root of its molar mass.
2. Gas Laws
Boyle’s Law
Boyle’s law states that the pressure of a gas is inversely proportional to its volume at constant temperature.
Mathematically, Boyle’s law can be expressed as:
P₁V₁ = P₂V₂
where P₁ and V₁ are the initial pressure and volume of the gas, and P₂ and V₂ are the final pressure and volume of the gas.
Boyle’s law can be explained by the KMT by considering the following:
- As the volume of a gas decreases, the gas particles become more crowded and collide with the walls of the container more frequently.
- This increased collision rate leads to an increase in the pressure of the gas.
Charles’s Law
Charles’s law states that the volume of a gas is directly proportional to its absolute temperature at constant pressure.
Mathematically, Charles’s law can be expressed as:
V₁/T₁ = V₂/T₂
where V₁ and T₁ are the initial volume and temperature of the gas, and V₂ and T₂ are the final volume and temperature of the gas.
Charles’s law can be explained by the KMT by considering the following:
- As the temperature of a gas increases, the average kinetic energy of the gas particles increases.
- This increased kinetic energy causes the gas particles to move faster and collide with the walls of the container more frequently.
- This increased collision rate leads to an increase in the volume of the gas.
Combined Gas Law
The combined gas law is a combination of Boyle’s law and Charles’s law.
Mathematically, the combined gas law can be expressed as:
P₁V₁/T₁ = P₂V₂/T₂
The combined gas law can be used to solve problems involving changes in pressure, volume, and temperature of gases.
3. Ideal Gases
An ideal gas is a gas that obeys the ideal gas law.
The assumptions of the ideal gas law are as follows:
- Gas particles are point masses with no volume.
- Gas particles do not interact with each other.
- The average kinetic energy of gas particles is proportional to the absolute temperature of the gas.
The ideal gas law can be derived from the KMT by considering the following:
- The pressure of a gas is proportional to the number of gas particles colliding with the walls of the container per unit time.
- The number of gas particles colliding with the walls of the container per unit time is proportional to the average kinetic energy of the gas particles.
- The average kinetic energy of the gas particles is proportional to the absolute temperature of the gas.
Therefore, the pressure of a gas is proportional to the absolute temperature of the gas.
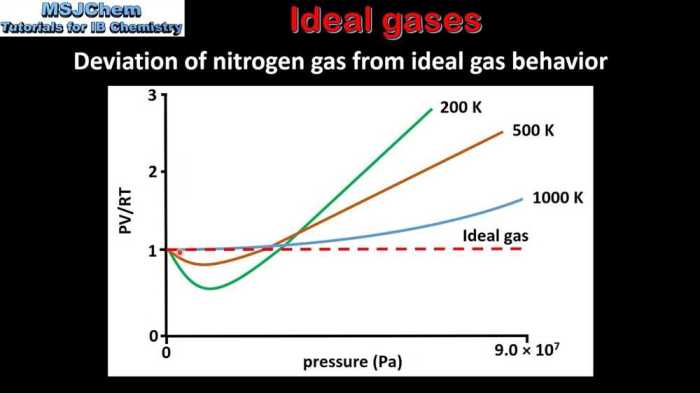
4. Real Gases
Real gases deviate from ideal behavior at high pressures and low temperatures.
The van der Waals equation is an equation of state that takes into account the deviations of real gases from ideal behavior.
The van der Waals equation is given by:
P = nRT/(V
- nb)
- a(n/V)²
where P is the pressure of the gas, V is the volume of the gas, n is the number of moles of gas, R is the ideal gas constant, T is the temperature of the gas, a is the van der Waals constant for attraction, and b is the van der Waals constant for repulsion.
The van der Waals equation can be used to predict the behavior of real gases at high pressures and low temperatures.
5. Gas Mixtures
A gas mixture is a mixture of two or more gases.
Dalton’s law of partial pressures states that the total pressure of a gas mixture is equal to the sum of the partial pressures of the individual gases in the mixture.
Mathematically, Dalton’s law can be expressed as:
Ptotal= P₁ + P₂ + … + P n
where P totalis the total pressure of the gas mixture and P₁, P₂, …, P nare the partial pressures of the individual gases in the mixture.
Dalton’s law can be used to calculate the partial pressure of a gas in a mixture.
6. Gas Effusion and Diffusion

Effusion, Chapter 14 the behavior of gases answer key
Effusion is the process by which a gas escapes through a small hole into a vacuum.
Graham’s law of effusion states that the rate of effusion of a gas is inversely proportional to the square root of its molar mass.
Mathematically, Graham’s law can be expressed as:
r₁/r₂ = √(M₂/M₁)
where r₁ and r₂ are the rates of effusion of the two gases, and M₁ and M₂ are their molar masses.
Diffusion
Diffusion is the process by which a gas spreads out from a region of high concentration to a region of low concentration.
Graham’s law of diffusion states that the rate of diffusion of a gas is inversely proportional to the square root of its molar mass.
Mathematically, Graham’s law of diffusion can be expressed as:
D₁/D₂ = √(M₂/M₁)
where D₁ and D₂ are the rates of diffusion of the two gases, and M₁ and M₂ are their molar masses.
General Inquiries
What is the kinetic molecular theory?
The kinetic molecular theory postulates that gases consist of tiny, constantly moving particles that collide with each other and the walls of their container.
How does Boyle’s law relate to the kinetic molecular theory?
Boyle’s law states that the pressure of a gas is inversely proportional to its volume at constant temperature. This can be explained by the kinetic molecular theory, as decreasing the volume of a gas increases the number of collisions between gas particles and the container walls, leading to increased pressure.
What is the ideal gas law?
The ideal gas law combines Boyle’s law, Charles’s law, and Avogadro’s law into a single equation: PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the ideal gas constant, and T is temperature.